Fall 2017: Building Livability
UTA researchers are creating a more sustainable, affordable North Texas for the future.
Skip to content. Skip to main navigation.
UTA researchers are creating a more sustainable, affordable North Texas for the future.
From carbon dioxide conversion to landfill mining, researchers at UTA are seeking viable alternative energy options.
Found in everything from space shuttles to dental fillings, composite materials have thoroughly infiltrated modern society. But their potential is still greatly untapped, offering researchers ample opportunity for discovery.
Within the particle showers created at the Large Hadron Collider, answers to some of the universe’s mysteries are waiting.
Model systems like pigeons can help illuminate our own evolutionary and genomic history.
UT Arlington's tiny windmills are bringing renewable energy to a whole new scale.
The stability of our highways, pipelines, and even manholes is reaching a breaking point.
Scientists believe they have discovered a subatomic particle that is crucial to understanding the universe.
UT Arlington researchers unlock clues to the human body’s most mysterious and complex organ.
UT Arlington researchers probe the hidden world of microbes in search of renewable energy sources.
Wounded soldiers are benefiting from Robert Gatchel’s program that combines physical rehabilitation with treatment for post-traumatic stress disorder.
Tiny sensors implanted in the body show promise in combating acid reflux disease, pain and other health problems.
Nanotechnology researchers pursue hybrid silicon chips with life-saving potential.
Biomedical engineers combat diseases with procedures that are painless to patients.

Photographs by Adam Voorhes
For something that weighs only about 3 pounds, the human brain looms large. Physiologically speaking, the body could not function without it, of course, but as the home of our memories, emotions, and thoughts, neither could we. When things go wrong with the brain manifesting as depression, dementia, epilepsy, stroke, and more—the collective result is an enormous health burden, with brain disorders affecting an estimated 1 billion people worldwide.
In April 2013, President Barack Obama launched the ambitious Brain Research Through Advancing Innovative Neurotechnologies, or BRAIN, Initiative, pledging $100 million for the first year alone toward the development of new tools to elucidate how the mechanisms of the brain actually work, ultimately shedding light on the underpinnings of neurological and psychiatric disorders and possible treatment approaches.
A year later, The University of Texas System picked up the call, launching the UT BRAIN Initiative to bring together the wide range of talent and expertise available across its 14 institutions and drive interdisciplinary and multi-institutional research collaborations. The UT System further cemented its commitment by pledging in November 2015 to lead the revolution in brain health as one of its nine Quantum Leaps.
“We really do think there is a revolution taking place in brain health, and we are determined to play a leading role in it,” says UT System Chancellor William H. McRaven.
Here at The University of Texas at Arlington, that revolution is evident in a variety of ongoing research projects, including two investigations of a phenomenon linked to traumatic brain injury in veterans and an analysis of the use of infrared light in cancer detection, memory retention, and other interventions.
Since 2015, UTA has received more than $6 million federal- and state-funded grants on brain research. Among the professors working on the topic are the following:

Associate Professor of Bioengineering

Professor of Computer Science and Engineering

Associate Professor of Mechanical and Aerospace Engineering

Professor of Bioengineering

Professor and Chair of Mathematics

Professor and Alfred R. and Janet H. Potvin Endowed Chair in Bioengineering

Research Assistant Professor of Bioengineering

Assistant Professor of Computer Science and Engineering

Associate Professor of Psychology

Assistant Professor of Earth and Environmental Sciences

Associate Professor of Computer Science and Engineering
Introduced in the late 1960s by a Hungarian physician, low-level light therapy (LLLT) has been heralded as a non-invasive way to promote wound healing and relieve inflammation and pain. Anecdotal evidence of its benefits abound, as do DIY products—from lamps and lightbulbs to helmets and handheld devices—promising consumers relief from afflictions as varied as arthritis and hair loss. In the brain, initial studies indicate that LLLT may have beneficial effects when it comes to stroke, memory loss, and mood disorders.
“Laser therapy has been used for years to relieve pain,” says Hanli Liu, bioengineering professor and a fellow of the American Institute for Medical and Biological Engineering. “But scientifically, people haven’t done very thorough, rigorous studies to understand or explain why.”
Lasers or light-emitting diodes can produce low-intensity light with wavelengths between approximately 700-1,100 nanometers. Moreover, they are thought to stimulate mitochondria, the “powerhouses” of cells, with one of their enzymes—cytochrome c oxidase (CCO)—converting the absorbed light into metabolic energy that cells can use. In August 2016, Dr. Liu and her collaborators published the results of a groundbreaking study that sought for the first time to quantitatively measure in living tissue how a low-level infrared laser stimulated CCO and increased hemoglobin oxygenation. The work was funded in part by a two-year seed grant from the UT BRAIN Initiative, which awarded a total of $5 million to 45 UT System research teams.
As detailed in Nature’s Scientific Reports, the researchers shone a 1,064-nanometer laser on participants’ forearms—a simpler system than the brain, which has multiple physical layers—in eight 55-second cycles, with a five-second break in between. Using near-infrared spectroscopy—a multicolor optical recording method that measures changes in light intensity at different colors to analyze blood flow and oxygenation—they demonstrated that CCO and oxygenated hemoglobin concentrations continued to increase over the duration of the experiment as the laser was administered. When Liu performed a similar experiment on participants’ foreheads, the results were consistent, demonstrating laser-induced increases in metabolic and hemodynamic activity in the human brain. That experiment was documented in a Journal of Cerebral Blood Flow & Metabolism study published online in February 2017.
Liu has focused her career on functional near-infrared spectroscopy (fNIRS), which she has also applied to cancer detection and functional brain imaging in vivo of human subjects, including UTA student veterans with post-traumatic stress disorder. Her frequent collaborator, UT Austin neuroscience Professor Francisco Gonzalez-Lima, is an internationally recognized leader in brain metabolic research on learning and memory functions and transcranial laser stimulation of the brain. Their combined expertise, along with that of their new collaborators from UT Southwestern and Texas A&M University, allowed them to secure a four-year, $2.85 million grant from the National Institutes of Health last August. Liu credits the research made possible by the UT BRAIN Initiative grant for helping them to win the highly competitive national NIH funding.
In the new study, the team is using dual-mode imaging modality—the 133-channel fNIRS optical brain imager and 64-channel EEG machine available at UTA’s state-of-the-art Shimadzu Institute for Research Technologies—to map how brain networks or circuits are modulated during and after transcranial infrared brain stimulation, or TIBS. In the early phase of the study, the researchers are exploring several fundamental questions through computer simulations and lab phantom studies: What thermal effects will TIBS generate on the human forehead and the brain, and how deep can the infrared light penetrate the brain and how much does it spread?
Liu notes that while Dr. Gonzalez-Lima’s behavioral studies have promisingly shown that TIBS is able to improve cognitive functions, there’s still much to be learned about the mechanisms underlying the technique. Still, she has high hopes for TIBS’ future.
“Once we understand those mechanisms, this technology could be available as a relatively non-invasive, low-cost, low-risk intervention device,” Liu says. “It has great potential in the future to improve people’s memory or, looking forward, maybe even relieve or reduce the progression of Alzheimer’s disease.”
According to the Centers for Disease Control and Prevention, traumatic brain injury (TBI) leads to more than 2 million visits to the emergency room each year. Most patients will experience mild TBI, which the CDC characterizes as “a brief change in mental status or consciousness.” While a return to normal cognitive functioning can be expected within three to six months in a majority of cases, the Department of Veterans Affairs notes that some studies indicate soldiers may still be experiencing problems two years after the initial injury.

Student veteran David Tyson participates in Hanli Liu’s clinical trial using fNIRS to map and measure cognitive dysfunction associated with PTSD.
Although blast-related brain trauma has been observed in military personnel since World War I, mild TBI has become the “signature wound” of the Iraq and Afghanistan campaigns, with an estimated 20 percent or more of these veterans returning home with a wide array of symptoms, from headaches and memory problems to impulsivity and depression. There are other wartime causes of mild TBI, including motor-vehicle accidents, but most of the injuries have been attributed to blast shockwaves generated by the increased use of improvised explosive devices on the battlefield.
However, despite the prevalence of these injuries and the potential for progressive neurodegenerative effects like Alzheimer’s disease and chronic traumatic encephalopathy, blast-related mild TBI is not well understood, as there are no physically observable wounds and the condition cannot generally be detected by standard imaging techniques. More fundamentally, how exactly these injuries occur in the brain and their precise physiological impacts remain unknown. But two UTA researchers, supported by grants from the Office of Naval Research (ONR), are investigating a promising lead: microcavitation.
It’s not yet clear what takes place in the brain as a shockwave generated by a high-order explosion passes through. One theory gaining traction is that, during the resulting pressure drop, micro bubbles form in the liquid areas of the brain. When these bubbles collapse—within five to 10 seconds in a lab setting—they produce a water jet that can impact surrounding tissues. It may not sound like much, but consider this: Cavitation is the phenomenon that causes the small pits observed in metal boat propellers.
Microcavitation is a phenomenon that requires liquid, so one of the challenges in investigating it is that the human brain is mostly made of water.
“Inside the brain, there are segments that are mostly fluidic—such as locations near the neurons or the cerebrospinal fluid under our skull—and there are also some small fluidic regions within the gray matter and white matter that include water, plus some other floating ions and biomolecules,” explains Ashfaq Adnan, associate professor of mechanical and aerospace engineering. “Hypothetically, cavitation can take place in any location, as long as the conditions for creating it are fulfilled.”
Through the development of a high-fidelity computation model, Dr. Adnan is investigating the impact of mechanical force on the neurons and their surrounding perineuronal nets—extracellular matrix structures that are more than 80 percent liquid. His work is funded by a two-and-a-half-year, $401,786 grant from ONR and UTA.
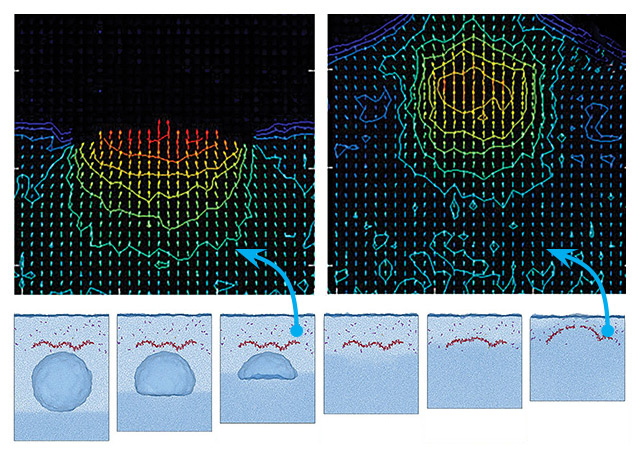
Ashfaq Adnan’s figures show the bubble collapse process and subsequent effect on the hyaluronan. From this, it can be inferred that when a shockwave passes through the cavitation bubble, the post-shock pressure gradually compresses its anterior side.
In July, Adnan and his postdoctoral associate, Yuan Ting Wu, published a study in Nature’s Scientific Reports that detailed their findings investigating whether the water jet released when a cavitation bubble pops can break the hyaluronan, a chief structural component of the perineuronal nets that, when damaged, is known to have some links with neurodegenerative diseases.
Using reactive molecular dynamics simulation, they modeled 12 events, using four bubble sizes (no bubble, 5 nanometers, 8 nanometers, and 10 nanometers) and three different shockwave velocities (3.6 km/s, 5.35 km/s, and 7.2 km/s). They then rebuilt the models two more times to statistically validate it, which resulted in 36 independent sets of simulations.
The team observed that the shockwave itself was not enough to break the hyaluronan; when no bubble is present, the hyaluronan is largely unaffected, regardless of the shockwave speed. However, as the front side of the bubble compresses from shockwave pressure, the bubble collapses at an even faster speed, creating stronger water jets. At the 3.6 km/s and 5.35 km/s speeds, a shockwave acting on the 5-nanometer bubble bends the hyaluronan. In the remaining sets, as the bubble size grows and/or the speed increases, the hyaluronan breaks, at times in multiple pieces.
Based on these findings, Adnan and Dr. Wu concluded that the larger the bubble, the stronger the jet—and the more damage done to the perineuronal net.
“The cavitation bubble, if it forms, will always collapse, and if we have shock-induced jet formation, this may or may not lead to damage of the extracellular matrix,” Adnan says.
He notes that he wouldn’t have been able to pursue this research without the top-notch computational power provided by the Texas Advanced Computing Center at UT Austin.
But even with that support, available computational tools limited the study to small-scale, small-duration events in the neuronal substructure. Moving forward, Adnan will use the evidence he’s gathered at the nanoscale and employ multiscale methods to develop a model that permits the team to examine what happens at an increased length of scale and duration.
The ultimate goal of the project is to build a complete model of an individual neuron.
“Then, we’ll have a better picture of what is going on and what could happen to the neuron and its surroundings.”
“I don’t want to stop at knowing what the problems are. We’re in the business of figuring out what could be done. That’s why I’m so excited by bioengineering programs like ours that are asking the question, ‘What can we do to impact society?’"
For Michael Cho’s microcavitation study, the first task was to establish whether the phenomenon could even occur in the brain. In spring 2015, the Alfred R. and Janet H. Potvin Endowed Chair in Bioengineering and his collaborators constructed phantom models of the brain—plastic molds filled with Jell-O. When shockwaves were passed through them in the lab, tiny bubbles did indeed form.
“The conclusion is that it is likely that these micro bubbles are being formed in the brain,” says Dr. Cho, who is a fellow of the American Institute for Medical and Biological Engineering.
That finding prompted a slew of questions about what was actually occurring. With the support of a three-year, $1.24 million grant from ONR, Cho and his team set out to record, monitor, and characterize the micro bubbles, following their trajectory in real-time from formation to collapse and assessing the damage done to surrounding brain cells.
To do this, they are engineering biomimetic brain tissue—which can mimic the major functions of real brain tissue—from mouse astrocytes and neurons. Using a controlled electrical discharge system (analogous to what happens with lightning and thunder), Cho generates shockwaves in the lab and then views through a microscope the cellular and subcellular responses stemming from the formation of micro bubbles. He can watch in real-time as the bubbles form and rise to the top, observing the impact they have as they hit the cells and pop.
The experiments have found that the force of the bubble collapse knocks some cells off the substrate, presumably killing them. Others become partially detached and start undergoing apoptosis, or programmed cell death.
In addition to examining the general effects of the micro bubbles on biomimetic tissue, the collaborative team—which includes researchers from Old Dominion University, Purdue University, and the UTA Research Institute (UTARI)—is focusing on how blood vessels could be impacted by microcavitation and how this might affect the blood-brain barrier.
In the brain, capillaries are lined with tightly packed endothelial cells, which form a semi-permeable wall and function as a sort of security system, preventing “foreign” substances—including toxic compounds and many medicinal drugs—from passing from the blood into the brain.
“If some of those micro bubbles are formed inside the vessel, that creates even more lasting problems,” Cho says. “If they collapse with that kind of force, it’s going to make your vessel leaky, allowing substances that are not supposed to enter the brain to diffuse out.”
The first challenge in tackling this region is the microfabrication of a biomimetic blood vessel approximately the width of a strand of hair from appropriate mouse cells. One of Cho’s doctoral students has spent the last year at UTARI creating, validating, and characterizing an initial model, then testing it for leakiness with alcohol, which is one of the few substances that can pass through the blood-brain barrier. The next step is to test the impact of micro bubbles to determine if and how such mechanical force could damage or destroy the cells, thus compromising the barrier.
Considering that the mechanisms behind mild TBI are still being explored and current imaging techniques cannot generally detect the damage, it perhaps comes as no surprise that there are no treatments for the injury itself.
“There are a limited number of FDA-approved drugs that are used to address symptoms, like [PTSD-related] nightmares, but the damage is done,” Cho says. “That’s very frustrating to me.”
But he and his fellow researchers see therapeutic promise in a polymeric compound known as poloxamer 188, which is FDA-approved for use as a blood thinner. While Cho believes the detached cells cannot be rescued, the thought is that the poloxamer could function as a molecule-sized Band-Aid, patching up the leaky spots while the partially detached cells work to repair themselves.
“I don’t want to stop at knowing what the problems are,” Cho says. “We’re in the business of figuring out what could be done. That’s why I’m so excited by bioengineering programs like ours that are asking the question, ‘What can we do to impact society?’ For us, it’s about improving human health. That’s the goal driving what we do.”
Researchers at The University of Texas at Arlington are exploring issues such as Alzheimer's disease, traumatic brain injury, developmental and learning disorders, anxiety, depression, and other serious brain-related conditions.
"With more than $6 million in new research grants since 2015 and a team of almost a dozen researchers focused on the brain, UTA is building a solid program to produce solutions to real-life problems associated with brain health," says Duane Dimos, vice president for research.
How the brain and eyes evolved
Chemical imbalances linked to autism, Alzheimer’s, Down Syndrome, and more
Link between fluctuating hormones and addiction in women
Function of D-amino acids in the brain
Understanding irrational thoughts about chronic pain
Treating depression among teenagers
Advising parents and medics about untreated concussions
Creating neuroscience guides to help parents engage children ages 5 to 10 in science, technology, engineering, and math concepts
Applying neurobiology and psychology to learning
Using RC21X program to test for sports-related head injuries
Robot-based systems assessing workers’ skills and learning
Determining how shockwaves injure the brains of soldiers in battle
Combining topology and machine learning to analyze complex brain data and images
Using mathematical models to screen for infant neurological diseases
Transcranial infrared brain stimulation tool to enhance cognitive function
Implantable in-line shunt flow monitoring system for readings of hydrocephalus
Portable brain imaging tool to screen encephalopathy in newborns
Deep brain stimulation devices to reduce pain
Near-infrared light to treat veterans with PTSD and traumatic brain injuries
Wearable device to help violent offenders manage their emotions